

- Cobalt chloride le chatelier lab install#
- Cobalt chloride le chatelier lab Offline#
- Cobalt chloride le chatelier lab free#
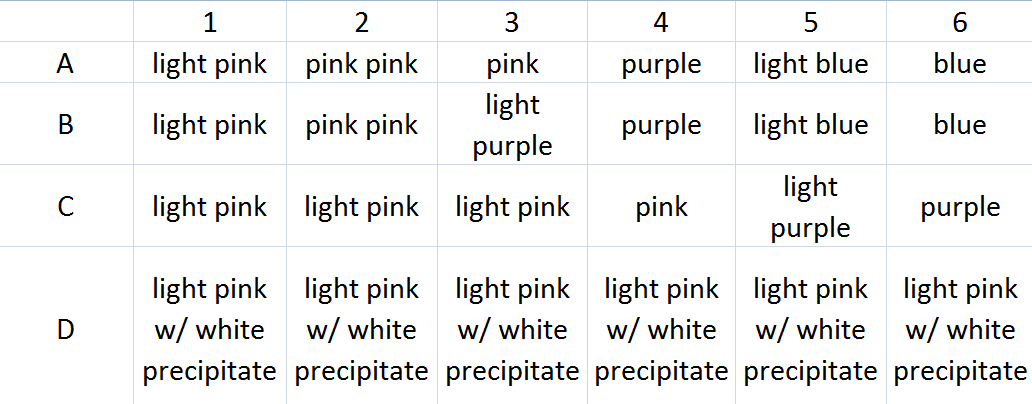
I can explain why the equilibrium shifts in a 3-5 sentence justification paragraph. LT 8.7 – I can define Le Châtelier’s principle and use this principle to predict how concentration, temperature, and pressure will impact a given equilibrium.Lecture 8.7 – Le Châtelier’s Principle II Heat or cool the system until you have perturbed the equilibrium.Cobalt (II) Chloride Equilibrium Co(H2O)62+(aq) + 4Cl-(aq) ⇌ CoCl42-(aq) + 6H2O(l) (Think-Write): According to LC’s Principle, why do you think the color changed? (Pair): Find out what your partner thinks (Share): Share your findings with the class. You can now change the temperature between 0 and 99 deg C. Right-click on the flask and choose “thermal properties”. Now do so in very small incremental steps Predict the effect of adding HCl to the reaction. Precipitate, and thus removes them from the solution.
Cobalt chloride le chatelier lab free#
Note by the above equation how Ag+ scavenges free chloride ions by tying them up in a Until the equilibrium has been shifted instead of a whole bunch at once. Now remove some of the free chloride ionsīy adding some silver nitrate by adding 1 mL amounts of the silver nitrate successively

Predict the effect of removing chloride ions. “pour” until you see a change, counting clicks to determine total volume added. Type in 1 for the volume to be transferred, and then keep clicking Take 12 M HCl solution from the stockroom and add 1mL increments until the equilibriumĬolor has changed. Be sure to include appropriate dilution factors. Use the equilibrium concentrations after each step to determine the K (equilibriumĬonstant) for the above equation. The molarity of each species is provided in the information Volume to be transferred in the space provided and then pour. Take 1 M CoCl 2 from the stockroom and add 25 mL of the solution to the emptyĮrlenmeyer flask by dragging 1 M CoCl 2 containing flask on to the empty flask.

Selected glassware or solution is provided in the side. Select 250 mL Erlenmeyer flask from the stockroom and see the information for the Solution, 12 M HCl solution, 6M AgNO 3 solution, glassware, and tools needed for this
Cobalt chloride le chatelier lab Offline#
Also, guidelinesįor the assignment available in problem description in offline interface and Cobalt LabĬheck the stockroom explorer, you have been provided with distilled water, 1 M CoCl 2 To load the assignment of offline version, select File Load Homework. Visit Virtual labs (watch the Introductory Video and Support Information to be familiar with the software) Online Simulation Interface Offline Simulation Interface Procedure: Part I: Effect of Concentration 1. Co(H 2 O) 6 +2 + 4Cl - CoCl 4 -2 + 6H 2 O pink blue Theory: LeChatlier’s Principle states that if a dynamic equilibrium is disturbed by changing the conditions such as temperature, concentration, the position of equilibrium moves to counteract the change. Help with this experiment you can contact me via email Purpose: To understand LeChatlier’s Principle by exploring the equilibrium reaction of the cobalt chloride under induced perturbations on the equilibrium.
Cobalt chloride le chatelier lab install#
Your computer, you may need to install the Java Plug-in for the Virtual Lab to run. Note that if you are installing the Virtual Labs in You can do this activity either online or offline.
Select the Equilibrium section under Virtual Labs. Cobalt Chloride and LeChatlier’s Principle


 0 kommentar(er)
0 kommentar(er)
